Tragedy struck when her 9-year-old daughter was killed in a car accident. Cain wanted to make a new start—to “chase my dreams”— and so she relocated from the Midwest to Washington state. She divorced and remarried, supported her new husband through his academic pursuits, and then, finally, went to college herself in 2011 as a returning-adult student. She started at Olympic College and finished at Puget Sound, majoring in biology with an emphasis in neuroscience, then earned an online master’s degree in microbiology and cell science from the University of Florida.
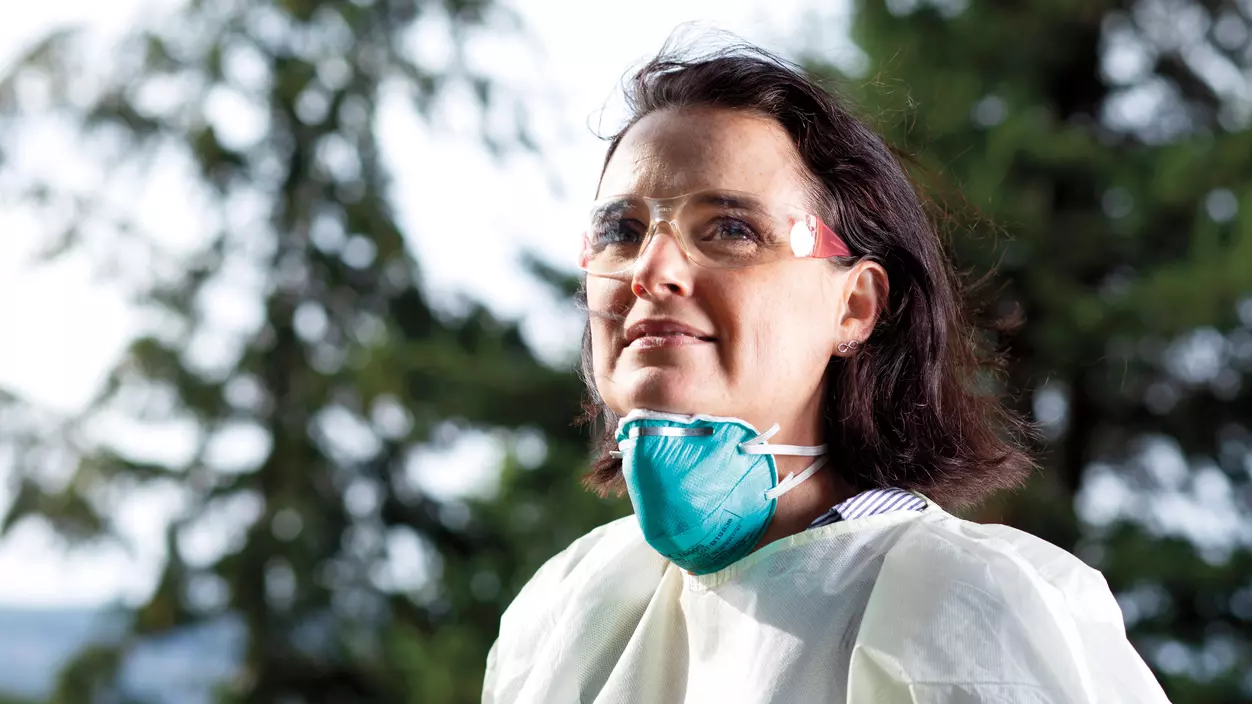
Today, Cain is a key player in efforts to treat COVID-19. She oversees human clinical trials of remdesivir—an antiviral drug showing promise in treating the disease—at Joint Base Lewis-McChord’s Madigan Army Medical Center. When she started in the role in August of last year, before the coronavirus pandemic hit, her work centered on the effectiveness of the three commercially available influenza vaccines. “I learned immediately how research really worked, how to recruit study participants, deal with the consent process, and keep track of everything,” she says.
Five months later, the coronavirus began its global spread, and Cain was recruited to lead a new study—one that would explore the safety and effectiveness of experimental medicines like remdesivir in treating COVID-19. She has organized and overseen it all, from getting the hospital approved for the study to ensuring correct protocols were followed and reporting deadlines were met.
For Cain, the job involves working with nearly every person in the medical setting. On an average day, she’ll gather consent from patients who qualify for remdesivir treatment, a lengthy and important process to ensure that patients fully understand the procedures and their possible outcomes. (Patients in the trial at Madigan include active-duty and retired military, as well as their dependents and beneficiaries.) She’ll also meet with researchers and physicians to check on patient progress, and ensure that adverse events are reported within the 24-hour window required by the relevant review boards. She tracks the patients, their treatments, and the results to report back to the relevant agencies.
Her team finished the first phase of the trial in May, along with nearly 70 other participating sites worldwide. “To see this small part—what we’re doing at Madigan—multiplied by all these teams across the world and all the data, it’s amazing,” Cain says. In total, more than 1,000 patients worldwide were given either remdesivir or a placebo for 10 days, and their recovery times were recorded. Those on remdesivir recovered, on average, four days sooner than those given a placebo. The results of the study were published in The New England Journal of Medicine on May 22—an astonishingly fast turnaround time for medical research. That same month, the Food and Drug Administration issued an emergency-use authorization for remdesivir to treat COVID-19.
In June, Cain and her team began work on the second phase of the study. Remdesivir was given to all COVID-19 patients, then supplemented with either a cytokine inhibitor (a drug commonly used to treat inflammatory diseases) or a placebo. The new phase meant Cain started from the beginning with a new group of patients and new procedures, protocols, and timelines to be reviewed and tasks to be assigned. That second phase concluded in August, with results expected to be published soon (“It is extremely fast—and changing the way research has traditionally been done,” Cain says). At press time, new patients were being enrolled in a third phase, to test the effectiveness of remdesivir plus interferon beta-1a (a drug typically used to treat multiple sclerosis) against COVID-19.